|
| Thread: The interesting part of Chemistry - Explained | |
|
keldorn

  
  
Promising
Known Hero
that casts green flames
|
 posted September 25, 2011 07:51 PM
posted September 25, 2011 07:51 PM |
|
|
The interesting part of Chemistry - Explained
Hello everyone!
I am currently studying to be a chemist engineer at my university, and, even after completing the firts of the 5 years of studies, I've already learned quite a lot of interesting facts about everyday science.
What I'd like to do in this thread is posting everyday incidents that can be examined easily by the general public, but ones that are not known very widely. I have a nice collection of such incidents so I'll be able to post one every 2 or 3 days or so. Let me just give you an example.
Making instant ice at 25°C!!
I'm quite sure that you have already seen videos like or similar to this one: The instant ice trick
Stunning eh? People's first feeling is that this is just a movie trick, but it's quite the contrary. It's pure physical chemistry.
As you probably know, solutions sonsist of solvent and solute. Most of the solvents' solubility increases at higher temperatures. This means that if, say, 100 units of water solves 30 units of salt for example at 25°C, it solves more, say, 50 units at 70°C. Most of the materials are like this, they prefer warm when solvating.
Sodium-acetate (later NaAc) is no difference either. If you heat water, it will be able to solve more NaAc. This solution however, because of the size of the Ac- ions, is metastable even at an oversaturated state.
If you put salt into some water and heat it, it will be able to solve more salt. However if it cools back down to 25°C, salt re-crystallizes into solid phase and stays unsolved at the bottom of the bottle, preventing the solution from being oversaturated. NaAc is not quite like this. It has the ability to stay oversaturated even at 25°C.
This solution however is metastable, which means that very small physical effect may cause it to start to crystallize. If you looked at the person holding the "hot ice", you might have noticed that he was extremely cautious putting the solution down, as a very small rezonance would have initiated the crystallation. (The chemical background behind this effect however is that because of the size of the Ac- ions, it needs coarse and harsh surface to stick to, which has bigger micro-holes on the surface than other materials. Glass is quite flat even under a microscope, [however not perfectly flat] so Ac- ions don't fit in its holes). When the man touched the solution, his finger provided harsh surface for the crystallation process to initialize itself. The NaAc crystals also have harsh surface, so the crystallation is auto-cathalithyc.
The newly created crystals and the remnants of the solution form a very viscous material, which pours very slowly (effectively can be considered as solid), something like wet sand.
And what does this have to do with hand-warmers? Well, chemistry has a law that's called Theorem of Le'Chatelier, which says that if an equilibrium state is disturbed by an unbalancing circumstance, the system reacts to this so as to reduce the disturbing effect. So what does this means? Remember, we needed to heat the solution to become oversaturated, this is because the system required more energy to be able to solve more solvant. The inverse process, the crystallation, according to the Theorem of Le'Chatelier, requires less energy, so the system gets rid of it in the form of heat production. To sum it up: solving extra NaAc requires heat from us, but re-crystallation provides the heat back.
Hand-warmers work the same way too: in a plastic bag, there's saturated NaAc solution plus some extra unsolved NaAc. When you boil the bag, the extra NaAc also goes into the solution. The inside of the bag, of course, is coated with an ultra-flat material so as to prevent unwanted crystallation. After boiling it up, it stays oversaturated for days unless you want it to start to crystallize. In this case, you can push a button, which impacts a harsh-surfaced material into the solution to initialize crystallation and thus heat production. After reboiling the bag, the device can be used again.
So this is it. I hope you enjoyed this one  . Remember, I'll be posting more and more (and then even more) like this in the following days. In the meantime, please feel free to comment, ask questions or make demands on the next topics . Remember, I'll be posting more and more (and then even more) like this in the following days. In the meantime, please feel free to comment, ask questions or make demands on the next topics  . See you, best wishes. . See you, best wishes.
____________
|
|
Tsar-Ivor

  
     
Promising
Legendary Hero
Scourge of God
|
 posted September 26, 2011 10:40 AM
posted September 26, 2011 10:40 AM |
|
|
Science is interesting if one is whole-heartedly willing to delve into our worlds remarkable secrets.
____________
"No laughs were had. There is only shame and sadness." Jenny
|
|
keldorn

  
  
Promising
Known Hero
that casts green flames
|
 posted September 27, 2011 01:52 PM
posted September 27, 2011 01:52 PM |
|
|
Superfluid or Supercritical phase. Under normal circumstances, there are 3 phases of materials that people are usually aware of, as they are quite trivial: gas, fluid and solid phases. All known materials can be brought to each and every of these phases if the correct circumstances are established. These circumstances depend on the temperature and pressure. Here's a phase diagram of water:

As you can see, under some circumstances, water can be turned into steam and ice as well. All 3 phases are possible on standard air pressure: 1 atm., as if you take a look at the vertical diagram, you can see that if you draw a horizontal line at a pressure value above the O-point, all 3 phases can be reached. In this case, luckily 1 atm. is above the O-point, so, just by modifying the temperature, you can change between phases.
An interesting fact is that carbon-dioxide has its own O-point below 1 atm pressure, so it can't be turned into fluid under standard circumstances, only on lower pressure levels (vacuum). Also interesting is that water, unlike most of the materials, increase in volume during freezing, so it has a left-turning solid-liquid border line.
At the O-point, all 3 phases are present and they form an equilibrium.
The phase diagram is by far not yet complete. At a certain point of pressure and temperature, the material starts to lose difference between liquid and gas state. If you has the possibility to watch such a process, you'd think you have fatigue. At the supercritical point, the difference completely disappears. The phase diagram of carbon-dioxide:

Superfluid materials have quite interesting properties. For example, polar solvents can solve apolar solutes, unlike normal fluid materials. For example, solving caffeine out of the coffee is done with supercritical carbon-dioxide: powderized coffee-water mixture is added to it and, strangely enough, it solves caffeeine only, after which carbon-dioxide is removed and viola! Coffeine-free coffee is ready. This is called supercritical extraction.
Finally, one last interesting facts: according to theory there are a lot more than 4 phases of materials, they just require so high pressure and temperature that they are not yet discovered.
____________
|
|
Tsar-Ivor

  
     
Promising
Legendary Hero
Scourge of God
|
 posted September 27, 2011 02:03 PM
posted September 27, 2011 02:03 PM |
|
|
Quote:
Finally, one last interesting facts: according to theory there are a lot more than 4 phases of materials, they just require so high pressure and temperature that they are not yet discovered.
Surely they wouldn't be actual phases, but sub-phases, like with colours  Blue, green & red are the main colours & everything else are 'sub-colours' Blue, green & red are the main colours & everything else are 'sub-colours'
____________
"No laughs were had. There is only shame and sadness." Jenny
|
|
Corribus


Hero of Order
The Abyss Staring Back at You
|
 posted September 27, 2011 10:59 PM
posted September 27, 2011 10:59 PM |
|
|
They are distinct phases because they have distinct structures. Consider the fifteen or so forms of ice. They are all "solid" but have distinct crystal structures and equilibria.
And your interpretation of color is very unscientific. 
|
|
Tsar-Ivor

  
     
Promising
Legendary Hero
Scourge of God
|
 posted September 28, 2011 09:38 AM
posted September 28, 2011 09:38 AM |
|
|
I don't pretend to know what I'm talking about 
(atleast I hope not  ) And besides I did the best I can in the 2 minuites I had to spare ) And besides I did the best I can in the 2 minuites I had to spare
____________
"No laughs were had. There is only shame and sadness." Jenny
|
|
Corribus


Hero of Order
The Abyss Staring Back at You
|
 posted September 29, 2011 04:32 AM
posted September 29, 2011 04:32 AM |
|
|
The point being that what we call "colors" are just human perceptions of various light wavelengths. By which I mean, there is no absolute, rigorous meaning of "blue".
____________
I'm sick of following my dreams. I'm just going to ask them where they're goin', and hook up with them later. -Mitch Hedberg
|
|
keldorn

  
  
Promising
Known Hero
that casts green flames
|
 posted September 29, 2011 10:57 PM
posted September 29, 2011 10:57 PM |
|
|
Flame colour of elements of the periodic table
Some of the metals from the periodic table have a very interesting property. They have different flame colours which can be used to identify them in their compounds.
The phisical background behind this can be found at the level of nucleons and electrons. As you probably know, electrons can be found on atomic orbitals, which are situated around the nucleon. The more orbitals are filled, the larger the atom radius. Each of these orbitals have an energy level. This is something like putting an iron ball on stairs: if you put the ball on the first stair, it won't break a coconut if it falls, because the level of energy wasn't high enough. Altough if you put the ball a few stairs higher, it will have enough energy to break it. Atomic orbitals are similar, however if you want to transfer an electron from a lower lever to a higher one then, just like if you had to push the iron ball up the stairs, you need to invest energy.
Under normal circumstances electrons perefer to be at minimal energy level possible. This means that they can go to orbitals closer to the nucleon by themselves, but never away from it if you don't invest the energy in (also the ball won't go up the stairs by itself). However, if you invest the energy, the electron will jump up. When you stop investing the energy, the electron goes back from whence it came. How can you invest energy? For example you can heat the material, that's what we'd like to do to see the flame colouring. Electrons can't produce heat so they must find another way to emit the energy. They do so via emitting light with a certain wavelength, that's what we see as flame colouring.
Elements with flame-colouring properties
Sodium: golden yellow (589 nanometer wawelength)
Potassium: pale violet (768 and 404nm)
Lithium: crimson red (671 nm)
Stroncium: crimson red (663,675 nm)
Calcium: pale red ( 620, 554nm)
Copper: green (many wavelength values)
Barium: pale green (many wavelengths)
Similar colour effects
There are some materials which also show the ssame effect, but we don't consider them flame-painters because that's just the way they burn. For example, wood has the classical orange, "fire-coloured" flame while methane, the main compound of natural gas burns with a blue colour. Trimethyl-borate has a natural flame colour of green.
Fluorescence has the same theory as flame-painting: some electrons recieve photon which are later emitted. This can be checked in UV-light: fluorescence is detected via seeing that typical neon-green glowing.
Chemi-luminescence of tin(II) and tin(IV)- ions These ions in acidic solutions provide tin(IV)-hydride (SnH4) if zinc is also present, which has a typical blue luminescence. This is used to detect tin ions in their compounds. The test is done the following way: The tin-ion solution, the acid and zinc are put in a bottle. A test-tube is filled with water than put into the solution so that the surface of the test-tube gets wet from the solution. The test-tube is put in a flame, and on the surface the solution of the tin-hydride starts to produce the blue luminescence.
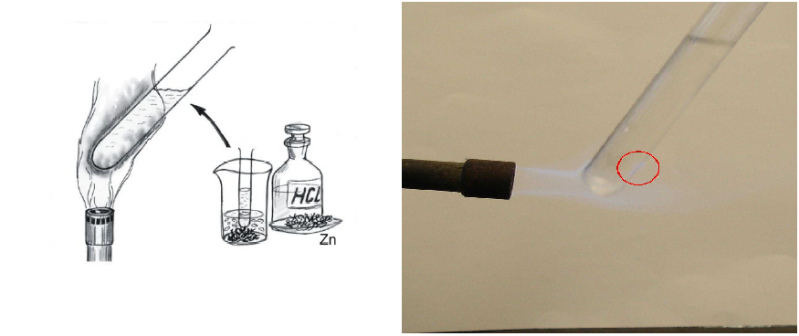
Sorry, there's just no better picture out there about this one 
____________
|
|
Corribus


Hero of Order
The Abyss Staring Back at You
|
 posted September 29, 2011 11:45 PM
posted September 29, 2011 11:45 PM |
|
Edited by Corribus at 03:12, 30 Sep 2011.
|
Quote:
Electrons can't produce heat so they must find another way to emit the energy.
Sure they can. If they couldn't, all (isolated) materials would be perfectly luminescent. In fact, in molecules anyway, radiating heat is by far the most efficient method of electronic relaxation. In atoms (gaseous, low pressure), however, there aren't vibrational states to make heat radiation efficient, which is why atoms tend to relax via photon emission.
____________
I'm sick of following my dreams. I'm just going to ask them where they're goin', and hook up with them later. -Mitch Hedberg
|
|
Shyranis

  
    
Promising
Supreme Hero
|
 posted September 29, 2011 11:50 PM
posted September 29, 2011 11:50 PM |
|
|
Colour, light, sound, heat.
All Radiation.
____________
Youtube has terminated my account without reason.
Please express why it should be reinstated on
Twitter.
|
|
Tsar-Ivor

  
     
Promising
Legendary Hero
Scourge of God
|
 posted September 30, 2011 09:29 AM
posted September 30, 2011 09:29 AM |
|
|
Ah the electro-magnetic-spectrum, I do love a-bit of ionised radiation.
Every energy transfer always produces heat, I this is the second law of thermodynamics, ergo you cannot have a fully 'efficient' energy tansfer (you can make them more efficient, 65% to lets say 70%, but it can never be 100%)
(first law is; energy is uncreatable & indistructable)
____________
"No laughs were had. There is only shame and sadness." Jenny
|
|
keldorn

  
  
Promising
Known Hero
that casts green flames
|
 posted October 08, 2011 03:38 PM
posted October 08, 2011 03:38 PM |
|
|
Quantum fluctuations Some laws of Phisics are all based on experiments, such as the pressure of gases. According to Phisics, gas particles move very quickly in the gaseous phase, colliding with both each other and the wall of the container. These collisions of course come with a force that the particle inflicts against the wall. This force, divided by the surface affected by this, gives the pressure of the gas. However, particles' movement are random. Who says that the particles of the air in a room can not move in the same direction via randomness? There's no such a law of nature, however the chances of this are tremendously low (fortunately  ) But how low it is exactly? Let's see it in an experiment: ) But how low it is exactly? Let's see it in an experiment:
Let's put 300 gas particles in a slide-valve (a barrel-shaped piston) and establish the piston so that the volume of the slide-valve is constant but large enough, say 1 m^3. Inside the device there's perfect vacuum if we ignore the pressure of the 300 particles. What's the chance that one particle will be in the first half of the slide-vlave? It's 1/2 or 50%. How about two being in the same 0,5 m^3? It's 1/4 or 25%. And 10 particles? It's 1/1024, which is approximately 0,1%. How about 300 particles all being in the first half? It's 1/(2^300) = 5*(10^-91) which is 0,0000...005% where the number of zeros is 89. And that's only 300 particles. Imagine this in a whole room full of air. We can safely say that the chance for all of the particles to be only in one half of the room even for a moment is zero.
So we saw that the laws of Physics allow the air to inflict double pressure to one half of the room, however the chances of this are unimaginably low. However, smaller disfunctions can happen. Fluctuation: the occurance of a very improbable quantum-event.
____________
|
| |
|
|





